Medical Device Design Starts with Human Factors
Our process and dedication to quality make us a perfect fit to develop new cutting edge product for the medical and biological fields.
Biology Analytics Machines
Therapeutic Devices
Surgical Tools and Equipment
Prosthetics and Assistive Wearables





TOOL follows a strict protocol with each and every client to ensure products we design will be safe and user friendly for medical workers or patients.
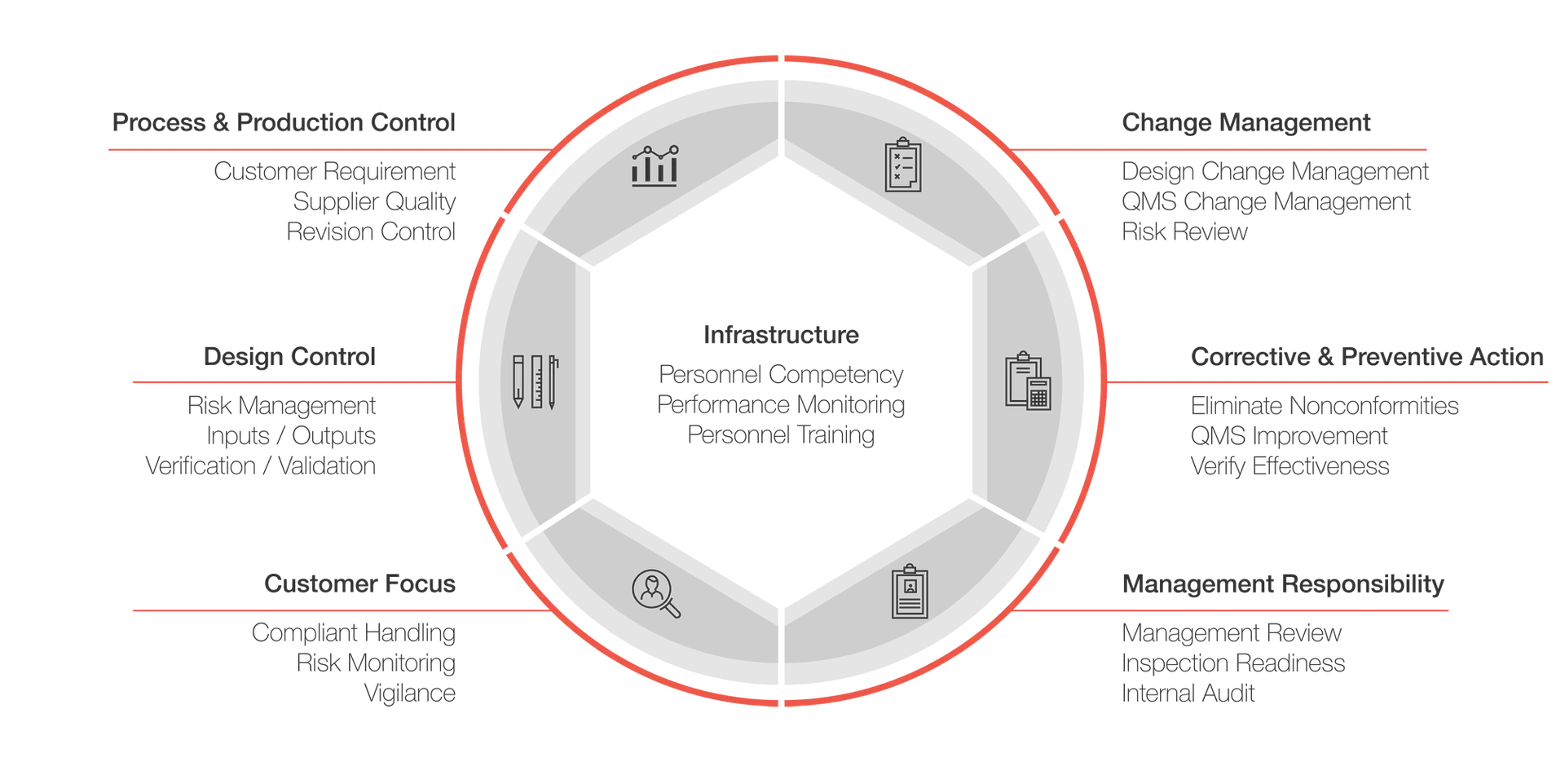
Quality Management System
Our Commitment to Excellence
Regulatory Strategy
User/Patient Safety
Testing Requirements
Regulatory Choices

Quality Assurance
Document Control
Traceability
Risk Management
Design History Files
Device Master Record
Accountability through QMS System
Reach out to us for our certificate +

Medical to Market
We practice clean product design through enhanced etiquette to find solutions that are economical and environmentally conscious. Every project is guided through our process with a goal to maintain the smallest footprint during and after the development.
UL and CE Certifications
Design Change Control Orders
Part Inspection
Production Documentation Packages
IEC 60601